Discovering the radioactivity

In 1896, Henri Becquerel discovered some strange radiation coming from uranium salts [Bec96]. Two years later, Pierre and Marie Curie isolated radium, a material far more radioactive than uranium and Marie Curie called this spontaneous emission of radiation “radioactivity”. The three physicists shared the Physics Nobel Prize in 1903 for the discovery of radioactivity. In 1899, Ernest Rutherford showed that two types of radiation exist, that he called alpha and beta, and in 1900 Villard found a third type of radiation coming from radium, that he called gamma. In 1902, Pierre and Marie Curie showed that beta radiation was nothing more than electrons. Later, it was determined that those electrons come out of the nucleus of the radioactive atoms.
The beta decay
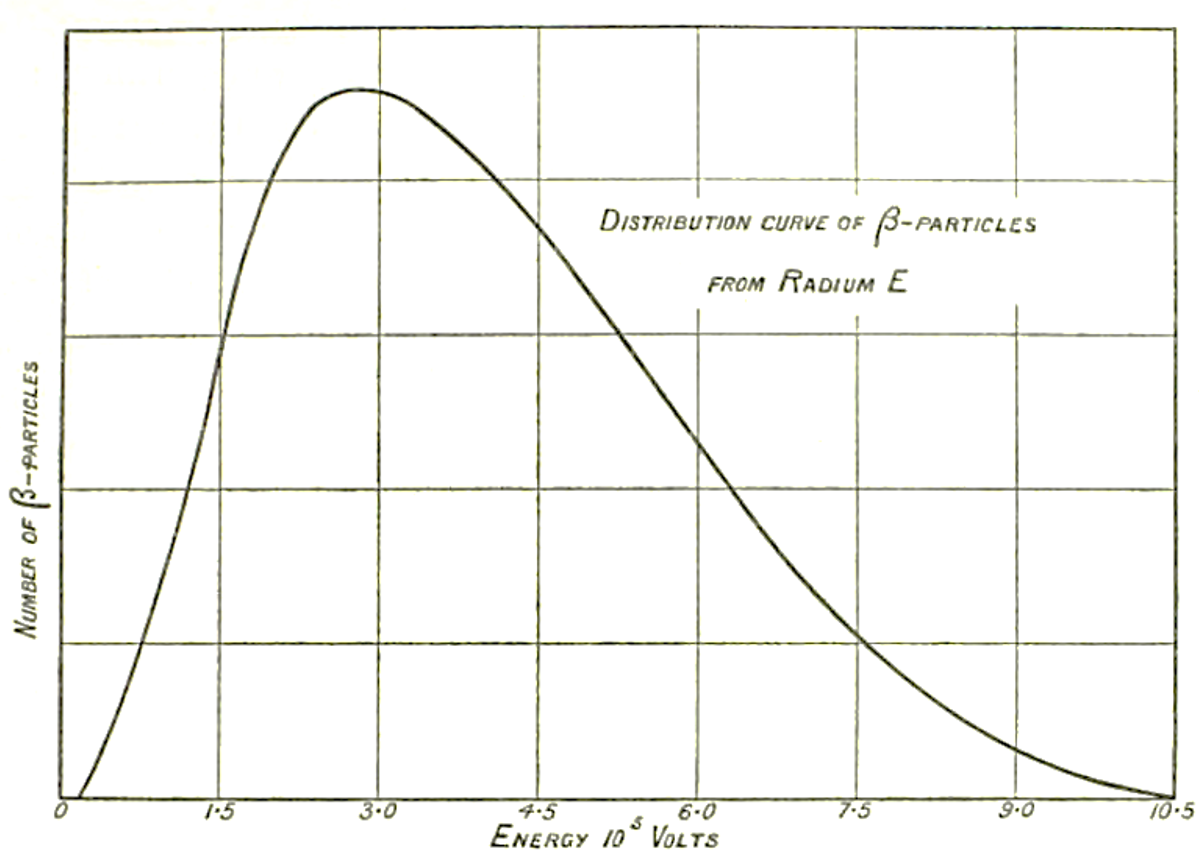
The beta decay was then identified as the reaction AZX → AZ+1X + electron, where a nucleus AZX decays in a nucleus AZ+1X, with the emission of an electron. Thanks to the conservation of momentum, the beta radiation (the electron), should have a fixed energy. But, after different experiments made in particular by Lise Meitner and Otto Hahn, James Chadwick, using a magnetic spectrometer and an electron counter, showed in 1914 [Cha14] that this was not the case: the electron energy spectrum was continuous, which means that its measured energy has any value up to a limit which is the energy it would have had if emitted alone. This result has not been immediately accepted since it could be due to experimental or theoretical artifacts which could simulate the observed behavior of the electron spectrum. We had to wait until 1927, when Charles Drummond Ellis and William Alfred Wooster [Ell27] made a decisive experiment on radium E (bismuth-210), using a calorimetric technique giving a direct proof that the electron spectrum was continuous. To face this contradiction, several important physicists, among them the famous Niels Bohr, suggested iconoclastically that energy was not conserved or was conserved “in the mean”.
An invented particle: the neutrino

This was the uncomfortable situation in 1930. The first idea of the neutrino came when Wolfgang Pauli tried a desperate rescue of “the energy conservation principle”. The 4th of December 1930, he declined an invitation to a workshop in Tübingen. But he sent to his colleagues a strange letter, which became famous, where he suggested that the electron is accompanied by a light, neutral, weakly interacting particle which takes away part of the energy [Pau30].
In February 1932, James Chadwick discovered the neutron [Cha32], but neutrons are heavy and did not correspond to the particle imagined by Pauli. Enrico Fermi used the term “neutrino” which was immediately accepted by the community. At the Solvay conference in Brussels, in October 1933, Pauli said [Pau33], speaking about “his” particle: “... their mass cannot be very much more than the electron mass. In order to distinguish them from heavy neutrons, mister Fermi has proposed to name them “neutrinos”. It is possible that the proper mass of neutrinos be zero… It seems to me plausible that neutrinos have a spin ½… We know nothing about the interaction of neutrinos with the other particles of matter and with photons: the hypothesis that they have a magnetic moment seems to me not funded at all.“ Soon after, Francis Perrin, analyzing the shape of the beta spectrum, shows that the neutrino mass has to be very much lower than the electron mass [Per33].
A theory and a name for the neutrino
At the end of 1933, incorporating the neutrino hypothesis, Fermi built the theory of beta decay, which describes the decay of a neutron into a proton, emitting an electron and a “neutrino” [Fer33] (later this “neutrino” will be identified as an “antineutrino”). This theory, extended to all weak interaction processes, is still valid today (excluding some modern improvements : we now know that nucleons are made of quarks and in the modern theory of beta-decay, a d quark is transformed into a u quark, producing the emission of a W boson which decays into an electron and an antineutrino).
At the same time, Carl Anderson discovered the positron, the first observed particle of anti-matter, clear confirmation of the theory of Paul Adrien Maurice Dirac and confirming the idea of the neutrino in the minds of Pauli and Fermi.
As early as 1934, Hans Bethe and Rudolf Peierls showed that the cross section (probability of interaction) between a neutrino and a proton should be extremely small: billions of times smaller than the one between an electron and a proton [Bet34]… and that the neutrinos would never be observed: “There is no practicable way of observing the neutrino”!
Further information
The online Symmetry Magazine published in March 2007 an article on the invention of neutrino.
During the conference on the History of the Neutrino (Sept. 5-7, 2018 in Paris) the Prehistory of the Neutrino was reviewed by :
- Allan Franklin (University of Colorado,USA) : here the slides the video of his talk and his contribution to the Proceedings.
- Cecilia Jarlskog (Lund University, Sweden) : here personal recollections and her contribution to the Proceedings.
References
| Author(s) | Title | Reference | Key-words | |
|---|---|---|---|---|
| Bec96 | H. Becquerel | 1) Sur les radiations émises par phosphorescence; 2) Sur les radiations invisibles émises par les corps phosphorescents | Comptes-Rendus de l’Académie des Sciences, 122 (1896) 420 (24 février 1896) & Comptes-Rendus de l’Académie des Sciences, 122 (1896) 501 (2 mars 1896) - Details in a paper, in French, by J.L. Basdevant The first paper is translated in English in « A. Pais, Inward Bound, Oxford University Press, 1986 | historical betadecay milestonebib overviewbib prehistbib |
| Bet34 | H. Bethe and R. Peierls | The neutrino | Nature 133 (1934) 532 | historical properties detection milestonebib overviewbib prehistbib discovbib |
| Cha14 | J. Chadwick | Intensitatsverteilung im magnetischen spektrum der beta-strahlen von radium B+C | Verhandlungen der deutschen Physikalischen Gesellschaft 16 (1914) 383 | historical betadecay milestonebib overviewbib prehistbib energybib plotbib |
| Ell27 | C.D. Ellis and W.A. Wooster | The Average Energy of Disintegration of Radium E | Proc. Roy. Soc. A 117 (1927) 109 | historical betadecay milestonebib overviewbib prehistbib oscbib energybib plotbib |
| Fer33 | E. Fermi | Tentativo di una teoria dell’emissione dei raggi beta | Ricerca Scientifica 4 (1933) 491 - Reprint in ”Enrico Fermi, Collected papers, vol. I, The University of Chicago Press” | historical betadecay milestonebib overviewbib prehistbib |
| Pau30 | W. Pauli | Dear radioactive ladies and gentlemen | Pauli to L. Meitner (in German) , English translation by K. Rieselmann, French translation | historical milestonebib overviewbib prehistbib energybib |
| Pau33 | W. Pauli | Discussion du rapport de M. Heisenberg « Structure et propriétés des noyaux atomiques » | 7ème Conseil Physique Solvay, Bruxelles, 1933, Gautier-Villars (1934) p. 324 | historical milestonebib overviewbib prehistbib energybib |
| Per33 | F. Perrin | Possibilité d’émission de particules neutres de masse intrinsèque nulle dans les radioactivités beta | Comptes-Rendus 197 (1933) 1625 | historical properties prehistbib propbib |
| Per34 | F. Perrin | La dissymétrie des spectres beta positifs et négatifs et la masse intrinsèque du neutrino ou ergon | Comptes-Rendus 198 (1934) 2086 | historical properties prehistbib propbib |

L’expression “theoretical artifact” mériterait peut-être quelques mots d’explication. Peut-être aussi que quelques mots sur la manière dont Francis Perrin a montré que la masse du neutrino était très inférieure à celle de l’électron seraient utiles.